Markus Ostermann1, Marko Piljevi´c1,2, Elahe Akbari 3, Prathamesh Patil 1, Veronika Zahorodna 4, Ivan Baginskiy 4, Oleksiy Gogotsi 4, Carsten Gachot 5, Manel Rodríguez Ripoll 2, Markus Valtiner 1,3 and Pierluigi Bilotto1,5
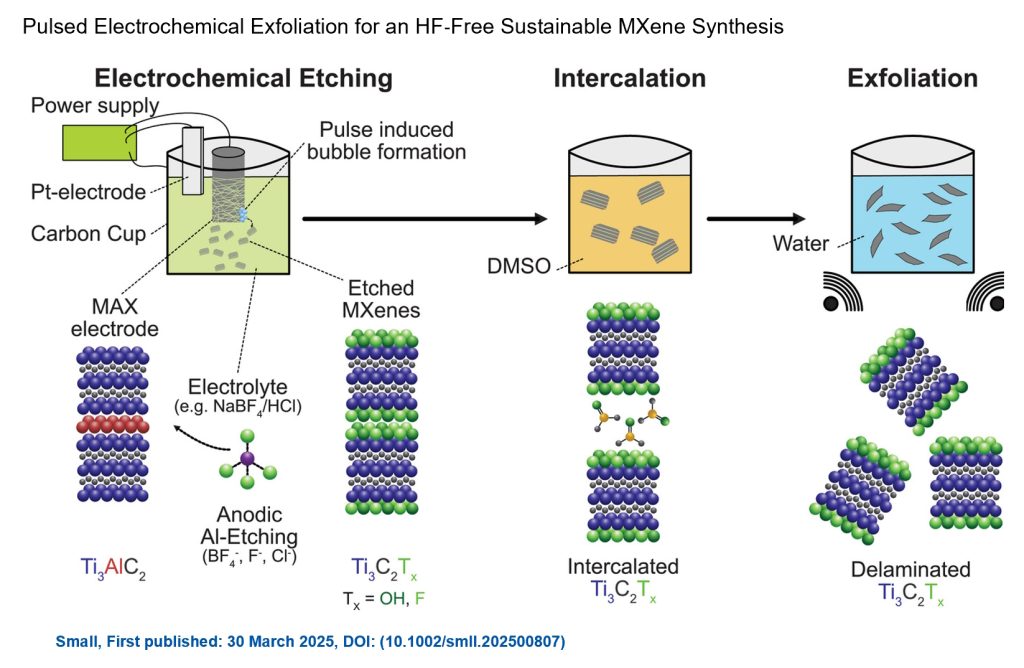
MXenes are a class of two-dimensional materials (2DM) with significant potential for industrial applications due to their exceptional properties and diverse compositions. However, achieving high etching efficiency during the synthesis process without using toxic, hazardous, or unsustainable chemicals poses a challenge. This study introduces a scalable electrochemical method for synthesizing MXenes. The innovative approach utilizes a non-toxic and sustainable sodium tetrafluoroborate/hydrochloric acid (NaBF4/HCl) electrolyte and enhances etching efficiency through cathodic pulsing via pulse voltammetry. The formation of hydrogen bubbles helps restore electrochemical activity and effectively aids in the removal of 2D sheets, enabling continuous etching with higher yields in situ. Specifically, yields of up to 60% electrochemical MXene (EC-MXene) are achieved from a single exfoliation cycle without any byproducts.
The quality of EC-MXene is excellent, with high purity confirmed through chemical mapping using scanning electron microscopy with energy dispersive electron spectroscopy (SEM/EDX) and surface termination analysis conducted with X-ray photoelectron spectroscopy (XPS) and, for the first time, low energy ion scattering (LEIS). Additional properties of EC-MXenes, such as the dimensions and adhesion energy of individual flakes, vibrational peaks, and interlayer spacing, are analyzed using atomic force microscopy (AFM), X-ray diffraction (XRD), Raman spectroscopy, and transmission electron microscopy (TEM), respectively. The pulsed electrochemical synthesis is crucial for reactivating the surface at the electrode interface, leading to enhanced exfoliation and quality of EC-MXenes. This advancement opens the door for the scaling up and environmentally friendly industrialization of MXenes.